Abstract
Introduction:
BCNU-based chemotherapy regimens (BEAM, BEAC) are viewed as the standard of care for conditioning prior to autologous hematopoietic cell transplantation (aHCT) in patients with lymphoma. The remarkable increase in the price of BCNU has prompted several transplant programs in Canada to review their preparative regimen prior to aHCT. Initial promising results of Bendamustine (Be) replacing BCNU in BEAM regimens (Be-EAM) contributed to its adoption at Hôpital Maisonneuve-Rosemont (HMR) where all cost are covered by a single payer government healthcare program. The goal of this study was to compare the efficacy, toxicity and pharmacoeconomic impacts of this substitution.
Methods
After approval from our institution Research Board and Ethics Committee, the files of 146 consecutive patients treated for lymphoma with aHCT at HMR between 01/2012 and 09/2016 were reviewed. From 01/2012 to 01/2015 patients were treated with BCNU containing preparative regimen (BEAM/BEAC) and afterwards with Be-EAM. Except for conditioning, indications for aHCT, eligibility criteria, treatment and supportive care were identical for the 2 groups according to SOPs of our FACT accredited transplant program. Clinical characteristics, parameters of engraftment, toxicities, post-transplant outcome and pharmacoeconomic impact of BCNU and Be containing conditioning regimens were compared and analyzed. Pharmacoeconomic evaluation included the cost of conditioning regimen, antibiotics and analgesics, blood products, total parenteral nutrition, growth factor, cost per day hospital (HSCT unit and intensive care unit (ICU) and length of stay.
Results
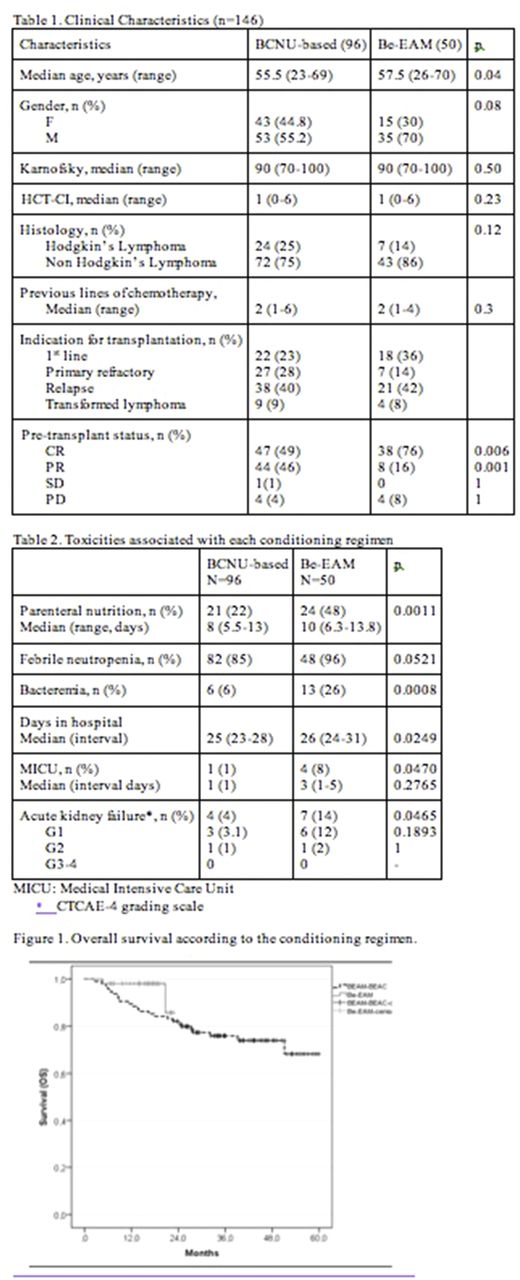
Clinical characteristics of patients receiving BCNU (n=96) and Be-EAM (n=50) are presented in Table 1. Age was slightly lower in the BCNU cohort and CR rate at transplant was higher in the Be-EAM group. The Be-EAM regimen was found to be more toxic (Table 2): platelet recovery was delayed requiring greater transfusion support in addition to a higher need for parenteral nutrition, incidence of renal failure, bacteremia and ICU transfers all leading to one additional day of hospitalization which impacted on total cost. There was no toxic death in the Be-EAM group and only one in the larger BEAM/BEAC cohort. With a median follow up (MFU) of 16 months and 34 months for Be-EAM and BCNU containing conditioning regimen, respectively, overall survival at 16 months (OS) appeared better in the Be-EAM group at 98% vs 86.3% (Figure 1. p=0.101) not reaching statistical significance. The significance of this data has to be interpreted in the context of the small cohort size and greater CR rate at the time of transplant in the Be-EAM cohort. Despite a substantial difference between conditioning regimen pricing in favor of Be-EAM (BEAM/BEAC median cost/patient Can$21 927 vs Be-EAM median cost/patient Can$11 330), the median cost saving per transplant was reduced to $5K when all additional toxicities were considered.
Conclusion
Substituting Carmustine for Bendamustine in the conditioning for aHCT of lymphoma patients increases toxicity and morbidity of the procedure, without impacting on transplant related mortality (TRM) suggesting that a specialized transplant team should be involved in the care of these patients to assure an increased awareness of potential toxicities. However, despite increased toxicity, OS are not inferior to the standard BCNU preparative regimens. Longer follow up and additional patients are required to confirm these results. Nonetheless, our data indicate that Be-EAM represents a reasonable, less expensive alternative to BEAM/BEAC albeit with slightly more morbidity.
Kiss: Otsuka: Membership on an entity's Board of Directors or advisory committees, Other: meeting attendance support, Research Funding; Alexion: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal